Rybelsus
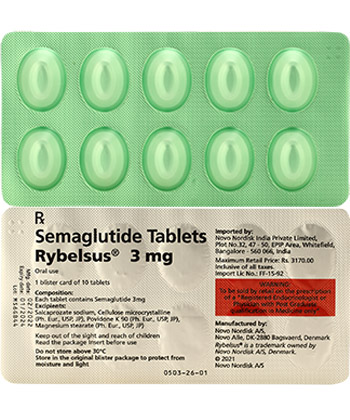
Rybelsus
- Rybelsus can be purchased at most pharmacies in Canada without a prescription.
- Rybelsus is used for the treatment of type 2 diabetes. It works as a glucagon-like peptide-1 (GLP-1) receptor agonist, helping to lower blood sugar levels.
- The usual dosage for Rybelsus starts at 3 mg daily, which may be increased to 7 mg or 14 mg based on the patient’s response.
- The form of administration is an oral tablet.
- The effect of the medication typically begins within 1 to 3 hours.
- The duration of action is around 24 hours.
- It is advisable to limit alcohol consumption while taking Rybelsus.
- The most common side effect is nausea.
- Would you like to try Rybelsus without a prescription?
Basic Rybelsus Information
- INN (International Nonproprietary Name): Semaglutide
- Brand Names Available in Canada: Rybelsus
- ATC Code: A10BJ06
- Forms & Dosages: Oral tablets (3 mg, 7 mg, 14 mg)
- Manufacturers in Canada: Novo Nordisk
- Registration Status in Canada: Prescription only
- OTC / Rx Classification: Rx (Prescription)
Safety Precautions Regarding Rybelsus in Canada
Rybelsus (semaglutide) falls under the strict regulations of Health Canada. It's essential for users to disclose any history of allergies or adverse reactions to GLP-1 receptor agonists to their healthcare provider before starting the medication.
High-Risk Groups for Rybelsus Use
Special attention is crucial for certain populations, particularly:
- Elderly Patients: This group may have increased susceptibility to side effects, especially gastrointestinal discomfort.
- Pregnant Women: Current data is insufficient to assess safety; medical advice is necessary before use.
- Indigenous Populations: With reported higher rates of diabetes, culturally tailored health resources may be important.
Close monitoring is vital for elderly patients and those from these high-risk groups. Health care providers should ensure that these individuals receive suitable care, making adjustments as necessary to their treatment plans.
Interactions with Activities Under Canadian Law
Patients considering activities such as driving or operating machinery should be aware of the potential for dizziness and nausea. It’s recommended to evaluate personal reactions to Rybelsus before engaging in any activities that require full mental alertness.
Q&A — Can I Drive After Taking Rybelsus in Canada?
Answer: This depends on how the individual reacts after taking the medication. If dizziness or nausea occurs, it’s best to refrain from driving. Always consult your healthcare provider for advice tailored to your situation.
Mechanism & Pharmacology
Understanding how Rybelsus works helps clarify its role in managing Type 2 diabetes. As a GLP-1 receptor agonist, Rybelsus enhances insulin secretion in response to meals. It also decreases glucagon levels, which play a key role in elevating blood sugar. This action helps lower blood sugar levels in a controlled manner. Clinical studies highlight Rybelsus's effectiveness in reducing HbA1c levels significantly, which is crucial for anyone managing diabetes. Moreover, patients often notice additional benefits, such as potential weight loss. Rybelsus is not just about blood sugar control; it aims to improve overall metabolic health.
Indications & Off-Label Uses in Canada
Rybelsus is approved by Health Canada for the management of Type 2 diabetes in adults. It is typically recommended as part of a comprehensive diabetes management plan that may include diet and exercise. However, some Canadian physicians are starting to prescribe Rybelsus off-label for weight management, even though this use isn't officially recognized. Patients seeking weight loss solutions may find this appealing, yet it's vital to approach off-label use with caution. Discussing individual health needs with a healthcare provider can yield the best outcomes when considering Rybelsus or alternatives for weight control.
Key Clinical Findings
Recent studies conducted between 2022 and 2025 in Canada and internationally have brought Rybelsus into the spotlight for its clinical efficacy. Research illustrates that patients using Rybelsus often experience considerable reductions in HbA1c levels, making it a valuable asset for diabetes management. These studies also report encouraging results regarding weight loss, further solidifying Rybelsus's reputation in the treatment landscape. Health Canada ensures ongoing monitoring for any potential adverse effects. This continuous supervision helps to keep the medication's safety profile updated. Patients can have peace of mind knowing that they are being monitored while on Rybelsus, allowing for timely interventions if any issues arise.
Alternatives Matrix
For those exploring options beyond Rybelsus, numerous GLP-1 receptor agonists are also available in Canada. Notable alternatives include Dulaglutide (Trulicity) and Liraglutide (Victoza), each offering different formulations and dosing regimens. When comparing Rybelsus to these alternatives, it can be helpful to consider the following pros and cons:
- Rybelsus: Oral administration, convenient dosing, potential for weight loss.
- Dulaglutide: Weekly injections, proven cardiovascular benefits.
- Liraglutide: Available in both diabetes and weight management formulations.
Common Questions from Canadian Patients
Patients often voice common concerns regarding Rybelsus, focusing on side effects, dosage schedules, and drug interactions. Questions frequently arise, such as "What should I do if I miss a dose?" and "How does Rybelsus interact with my current medications?" These inquiries are crucial, as proper understanding can enhance adherence and improve therapeutic outcomes. Health Canada and various local health offices have created accessible resources to aid patients in finding the answers they need. Patients are encouraged to use these resources and consult healthcare professionals to address their concerns and gain a comprehensive understanding of Rybelsus and its role in diabetes management.
Suggested Visual Content
Visual content can significantly enhance understanding and access to Rybelsus, particularly when navigating the complexity of drug plans across Canada.
Infographics on Provincial Drug Plan Coverage
How well do Canadians know their provincial drug plans? Infographics can simplify this by illustrating:
- Eligibility criteria for Rybelsus coverage
- Application processes
- Necessary documentation for access
Clear visuals are vital for enhancing patient education, ensuring they feel empowered in accessing Rybelsus. With the right infographic, the sometimes intimidating world of provincial drug plans becomes approachable.
Canadian Pharmacy Purchase Flowcharts
Confusion often surrounds the prescription and purchase process. Flowcharts serve as a practical tool to clarify the steps. They can outline:
- Consultation with healthcare professionals
- Obtaining a Rybelsus prescription
- Steps for purchase at pharmacies—whether online or in-person
These flowcharts simplify the journey from prescription to purchase, making it easier for patients to navigate and understand the process. Effective visual content not only engages but educates, which is crucial for patient empowerment.
Registration & Regulation
Ensuring the safety and efficacy of Rybelsus is critical, and Health Canada plays a pivotal role in this process. The drug must meet stringent approval requirements.
Health Canada Approval
Rybelsus received approval from Health Canada following a rigorous evaluation of clinical data. This means:
- Safety and efficacy studies support its use for diabetes management
- Monitoring for adverse reactions and side effects is mandated
This approval assures healthcare professionals and patients about the reliability of Rybelsus.
DIN Number and Labelling Requirements
All medications sold in Canada require a Drug Identification Number (DIN) to ensure proper tracking. Rybelsus follows these regulations and is labeled in compliance with Health Canada standards. These measures ensure patients receive quality medications with clear dosages and usage guidelines. Proper labelling includes:
- Clear dosing instructions
- Possible side effects and warnings
Rybelsus being well-registered and labelled enhances trust in the pharmaceutical system in Canada, ensuring that patients and healthcare providers have all necessary information readily available.
Storage & Handling
Knowing how to store medications properly can significantly impact their efficacy. For Rybelsus, standard storage guidelines make it user-friendly.
Standard Canadian Household Conditions
Rybelsus can be conveniently stored at room temperature, from 15ºC to 30ºC. Keeping it in its original packaging helps safeguard against moisture. Simple storage tips include:
- Store away from direct sunlight
- Avoid humid areas, like bathrooms
Cold-chain Requirements (where applicable)
Unlike medications requiring extreme temperature controls, Rybelsus does not need cold storage, making it easier for patients. This lack of stringent cold-chain requirements simplifies daily medication management.
Guidelines for Proper Use
Proper use of Rybelsus can significantly enhance its effectiveness. Education from pharmacists is crucial in guiding patients through best practices.
Canadian Pharmacist Guidance
Pharmacists are essential in educating patients on how to take Rybelsus effectively, including addressing potential side effects. This includes:
- The importance of taking Rybelsus in a fasted state
- Adherence strategies for long-term success
Provincial Health Authority Recommendations
Each province has tailored guidelines for dispensing Rybelsus. These recommendations may detail:
- Monitoring patient response
- Dose adjustments based on concurrent medications
Consulting local health authorities and aligning with regional guidelines ensures that patients not only obtain Rybelsus but also use it safely and effectively, enhancing the overall healthcare experience.
| City | Region | Delivery Time |
|---|---|---|
| Toronto | Ontario | 5–7 days |
| Vancouver | British Columbia | 5–7 days |
| Montreal | Quebec | 5–7 days |
| Calgary | Alberta | 5–7 days |
| Edmonton | Alberta | 5–7 days |
| Ottawa | Ontario | 5–7 days |
| Winnipeg | Manitoba | 5–9 days |
| Halifax | Nova Scotia | 5–9 days |
| Victoria | British Columbia | 5–9 days |
| Quebec City | Quebec | 5–9 days |
| Regina | Saskatchewan | 5–9 days |
| St. John's | Newfoundland | 5–9 days |